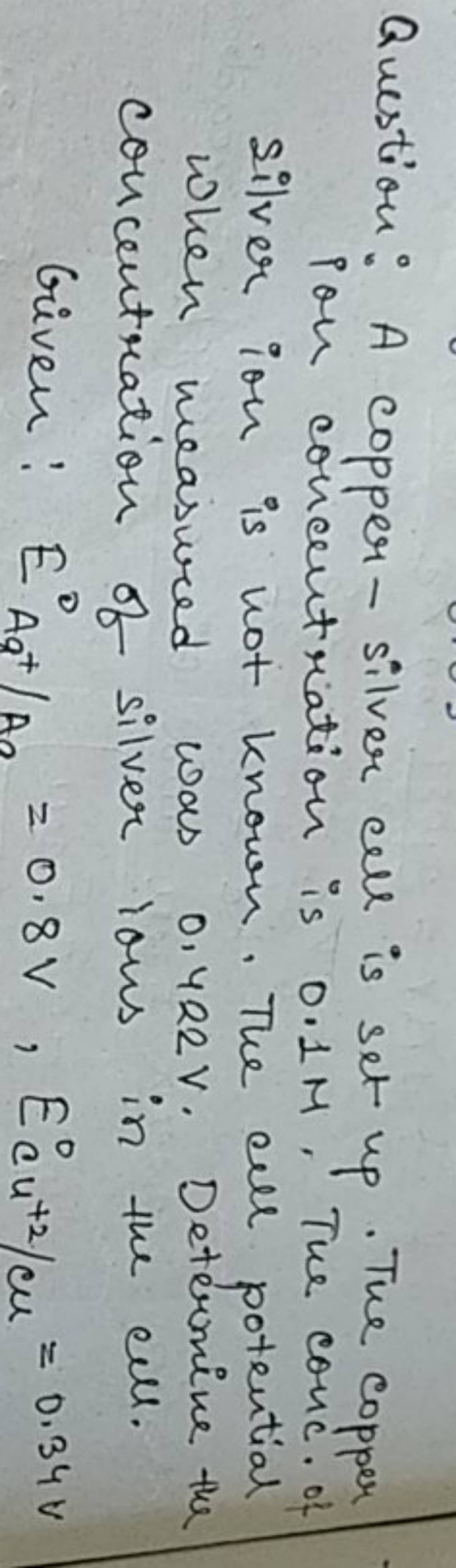Solve this: 8 A Copper-Silver cell is set up The copper ion - Chemistry - Electrochemistry - 12485096 | Meritnation.com

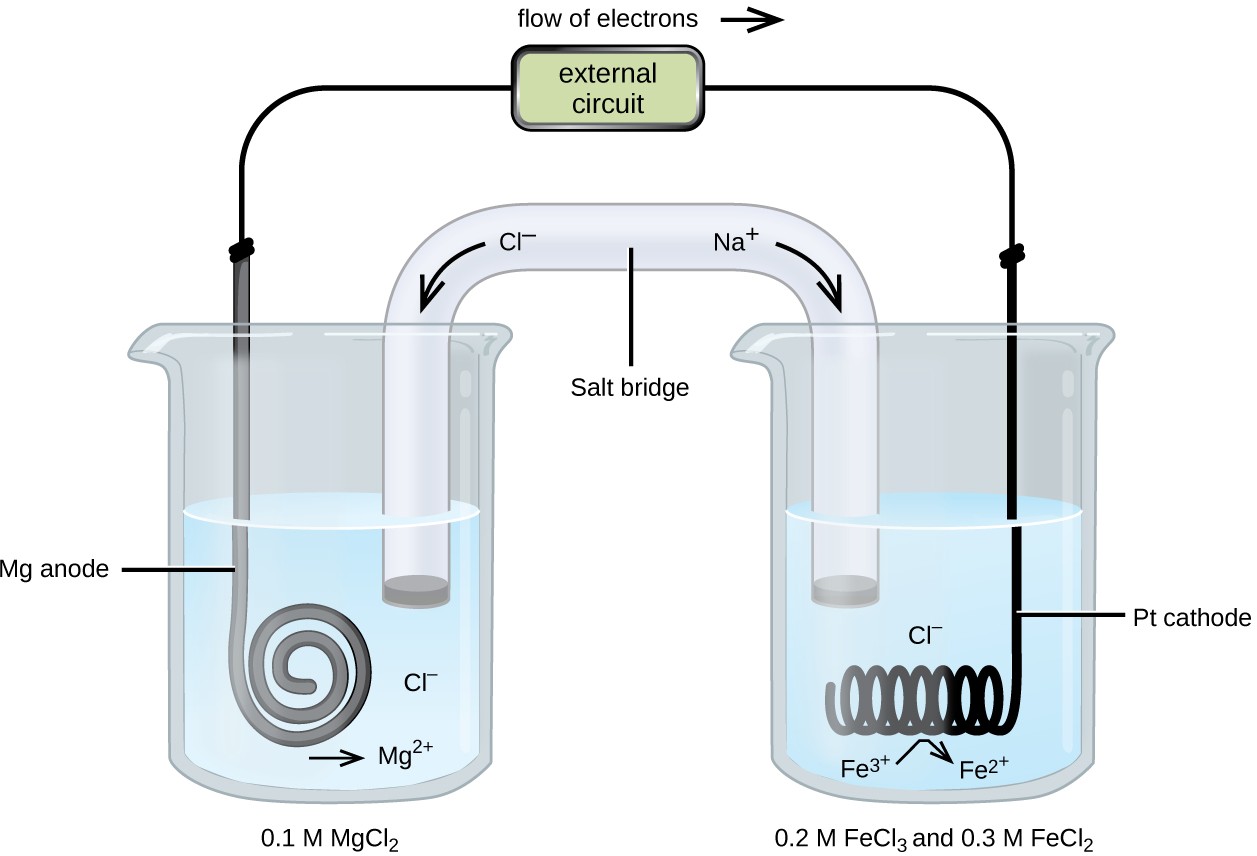
7. of A copper silver cell is setup. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. The cell potential measures 0.422V. Determine the

SOLVED: The Cu2+ ion concentration in a copper-silver electrochemical cell is 0.5 M. If Eo (Ag+/Ag) = 0.9 V, Eo (Cu2+/Cu) = 0.34 V, and cell potential (at 25°C) = 0.422 V,

HULUSUL PURCULUI 13.A Copper-silver cell is up. The copper ion concentration in it is 0.10M.The concentration of silver is not known. The cell potential measured is 0.422V.Determine the concentration of silver ion
A copper-silver cell is set up. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. - Sarthaks eConnect | Largest Online Education Community
BIM objects - Free download! Copper-Silver Ionization System - CSI - 2 Flow Cell 2 Controller Rack Mount - 2F2C | BIMobject

A copper-silver cell is set up. The copper ion concentration in it is 0.10 M. The concentration of silver - Brainly.in

7. of A copper silver cell is setup. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. The cell potential measures 0.422V. Determine the

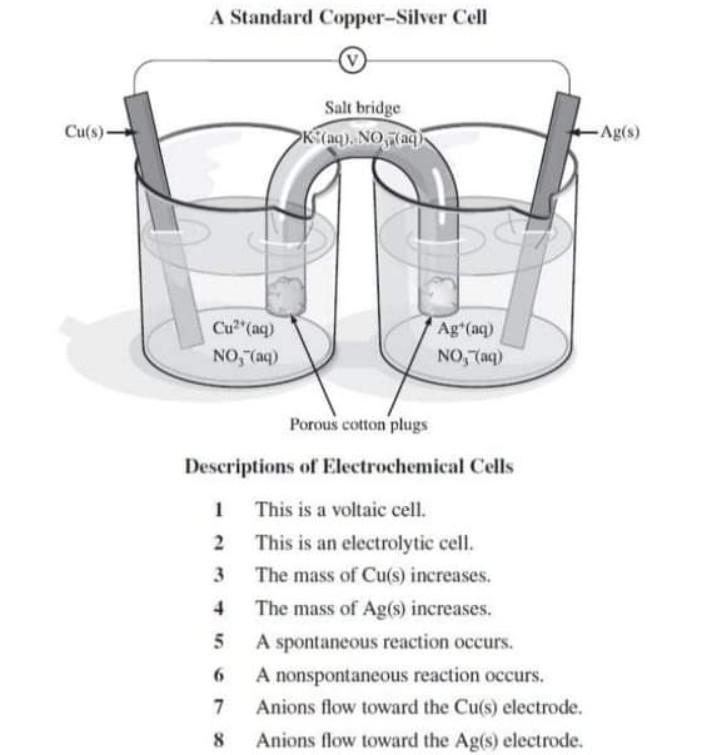
SOLVED: A copper-silver cell is set up. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. The cell potential is measured as 0.422 V.
A copper-silver cell is set up. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. - Sarthaks eConnect | Largest Online Education Community
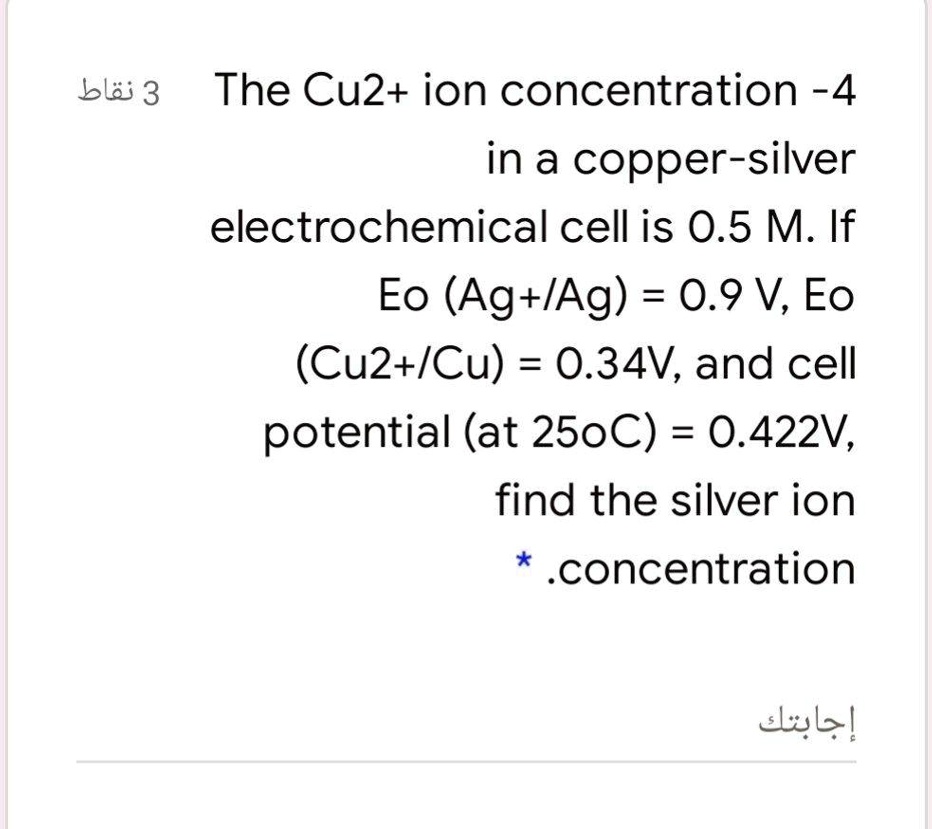
SOLVED: The Cu2+ ion concentration in a copper-silver electrochemical cell is 0.5 M. If Eo (Ag+/Ag) = 0.9 V, Eo (Cu2+/Cu) = 0.34 V, and cell potential (at 25°C) = 0.422 V,

The electrochemical cell for EMF measurement of chains of type (4.1)... | Download Scientific Diagram